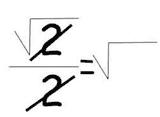-1.7475645946331821
Charged and Ready
An ion is just another way to say that an atom is charged. It either has extra electrons, making it negatively charged, or it is missing electrons, making it positively charged. When you put a bunch of these ions together, sometimes they will form a very ordered structure called an ionic solid.
The Structure of Salt

A great example of an ionic solid is a crystal of salt. Have you ever wondered what makes all those little atoms in salt stick together? Salt is made up of Sodium ions that have a +1 charge and Chloride ions that have a -1 charge. So, salt is held together by the attraction between the Sodium and Chloride ions because they have different charges. It is also kept from collapsing on itself by the repulsion of the same charged ions. When it is all put together, the ions line up to make a nicely structured three dimensional lattice.
Packed with Energy
Since these ionic solids are stuck together, it takes energy to break them apart. This energy is called lattice energy.
Now, suppose we wish to know what this lattice energy is for a given crystal. A good place to start would be to find the energy of a single ion in that crystal. To find this, we would need to know the sum of all the charges that act on that ion.
The Model for Success
Erwin Madelung(1881-1972), a German Physicist, tackled this problem in the early 20th century. He managed to calculate what is known as Madelung’s constant using the NaCl crystal known as rocksalt.
By defining a grid system with the origin at (0,0,0), he applied alternating charges at all the points (j,k,l) where j, k, and l are all whole numbers. This is a good model of what is going on in the crystal. The total charge at the origin, or the energy on that single ion, would have to be:
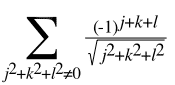
How Strong is Salt?
Using this mathematical model, Madelung calculated the energy of a single ion to be -1.74756495, known as Madelung’s constant. Then, using Coulomb’s Law, he determined the lattice energy of the entire crystal.
This theoretical lattice energy ended up being much larger than what was experimentally observed, so there was some concern about how well the model actually worked. As it turns out, the different answers were just caused by imperfections in the crystals.
Results!
Madelung’s constant can be used to determine the lattice energy for any crystal as long as you know the distance of an ion in the crystal to its nearest neighbor and the charges of all the ions in the crystal. It is because of Madelung that we can calculate how much energy is needed to break apart ionic crystals.